Materials Metals and Non-metals Class 8 Important Questions and Answers
Important questions of Class 8 Science Chapter 4 Materials Metals and Non-metals is given below. These important questions will help students while preparing for the exam. Practising these important questions will analyse their performance and work on their weak points. Score well in exam of Class 8 Science by going through these important questions. Students of Class 8 can download important questions of Class 8 Science Chapter 4 Materials Metals and Non-metals PDF by clicking the link provided below.
Important Questions of Class 8 Science Chapter 4 Materials Metals and Non-metals
Here you can get Class 8 Important Questions Science based on NCERT Text book for Class 8. Science Class 8 Important Questions are very helpful to score high marks in board exams. Here we have covered Important Questions on Materials Metals and Non-metals for Class 8 Science subject.
Very Short Answer Questions
1. How many types of materials are there?
Answer: There are two types of materials:
(i) Metals (ii) Non-metals
2. You are given following materials. Classify them into metals and non‑metals Iron, Coal, Sulphur, Aluminium and Copper.
Answer: Iron, aluminium and copper are the metals. Coal, sulphur are non-metals.
3. How can you distinguish metals from non‑metals?
Answer: Metals can be distinguished from non-metals on the basis of their physical and chemical properties.
4. Name two physical properties.
Answer: (i) Lustre (ii) Hardness.
5. What do you mean by malleability?
Answer: The property of metals by which they can be beaten into thin sheets is called malleability.
6. Coal and pencil lead do not show the property of malleability. Can we call them metal?
Answer: No, they are not metals.
7. What do you mean by conductivity?
Answer: The metals allow the electricity to pass through them. This property of metals is called conductivity.
8. What is ductility?
Answer: The property of metals by which they can be drawn into wires is called ductility.
9. What do you mean by sonority?
Answer: The property of metal, to produce ringing sound is called sonority.
10. Why are metals called sonorous?
Answer: Since metals produce ringing sounds, they are called sonorous.
11. Which materials are called non‑metals?
Answer: The materials which are not sonorous and are poor conductor of heat and electricity are called non-metals.
12. Name two metals which can be cut with a knife.
Answer: Sodium and Potassium.
13. Write the name of a metal which is found in liquid state at room temperature.
Answer: Mercury.
14. What is rust chemically known?
Answer: The rust is chemically known as Iron III oxide.
15. What happens when a metal reacts with oxygen?
Answer: The metal oxide is formed when metal reacts with oxygen.
Metal + Oxygen → Metal oxide.
16. What is the nature of metal oxide?
Answer: The Metal oxides are basic in nature.
17. What happens when a solution of metal oxide is tested with (i) blue litmus and (ii) red litmus?
Answer: (i) No change in the colour of blue litmus.
(ii) The red litmus changes into blue colour.
18. Give a chemical equation when iron reacts with oxygen.
Answer: Iron (Fe) + Oxygen (O2) → Fe2O3 (iron oxide)
19. Name the product formed by the reaction of sulphur and oxygen.
Answer: Sulphur dioxide gas.
20. Write the balance chemical equation when sulphur dioxide is dissolved in water.
Answer: Sulphur dioxide (SO2) + Water (H2O) → Sulphurous acid (H2SO3)
21. What is the effect of sulphurous acid on blue litmus?
Answer: Blue litmus turns red.
22. What is the nature of non‑metal oxides?
Answer: Mostly non-metallic oxides are acidic in nature.
23. What happens when sodium reacts with water?
Answer: Sodium is highly reactive metal. It starts to burn in water to form sodium hydroxide.
24. Write a balanced chemical equation of the reaction that takes place between water and sodium.
Answer: 2Na + 2H2O → 2NaOH + H2
25. Why is sodium stored in kerosene?
Answer: Sodium reacts very vigorously with oxygen and water and catch fire. Therefore, it is stored in kerosene.
26. Name a non‑metal which is kept in water.
Answer: Phosphorous is stored in water.
27. What happens when a metal reacts with acids?
Answer: Metal generally reacts with acids to give hydrogen gas.
28. What happens when a non‑metal reacts with acid?
Answer: Non-metals do not react with acids.
29. Does metal also react with bases?
Answer: Metals also react with some bases like sodium hydroxide and produce hydrogen.
30. What do you mean by displacement reaction?
Answer: The reaction in which a more reactive metal can replace a less reactive metal but a less reactive metal cannot replace a more reactive metal is called displacement reaction.
31. Give one example of displacement reaction.
Answer: When copper sulphate solution reacts with zinc then it displaces copper.
CuSO4 (Copper sulphate) + Zn(Zinc) → ZnSO4 (Zinc sulphate) + Cu (Copper).
Short Answer Type Question
1: Differentiate between metals and non-metals.
Answer:
| Metals | Non-metals |
| These are solids at room temperature except mercury | These exist in all three states |
| These are very hard except sodium | These are soft except diamond |
| These are malleable and ductile | These are brittle and can break down into pieces |
| These are shiny | These are non-lustrous except iodine |
| Electropositive in nature | Electronegative in nature |
| Have high densities | Have low densities |
2: Why we use aluminium foil to wrap food items?
Answer: The property of metals by which they can be beaten into thin sheets, is called malleability. Aluminium is a metal. Aluminium foils are made by using this property of aluminium. They keep food items warm and prevent them from getting contaminated.
3: Why can’t we store lemon pickles in an aluminium container?
Answer: As we know metals react with acids and produce metal salts and hydrogen gas, aluminium is a metal and lemon contains citric acid. So if we store lemon pickle in an aluminium utensil after some time utensil metal will get corroded due to reaction and lemon pickle inside will not be fit for human consumption.
4: Explain malleability in metals and non-metals.
Answer: The property of metals by which they can be beaten into thin sheets is called malleability. This is the characteristic property of metals which is exploited to make silver foil for decorating food items and aluminium foil to store food items.
Non-metals do not show this property, on beating a coal or wood they get break down into small pieces, thus we can say that metals are malleable and non-metals are not malleable.
5: Explain ductility in metals and non-metals.
Answer: The property of metals by which it can be drawn into wires is called ductility, thus we can see aluminium or copper wires around us. Non-metal do not show this property so we never see plastic wires around us, they are not ductile.
6: Why there is difference in sound on dropping a metal coin and a piece of coal?
Answer: This is because metals produce ringing sound and are called sonorous while non-metals do not show this property.
7: State some of the chemical properties of metals.
Answer:
Reaction with oxygen:
Metals react with oxygen to form metallic oxides. These are basic oxides because they react with water to form bases.
Eg. Magnesium burns in air to form magnesium oxide.
2 Mg + O2 → 2 MgO
Reaction with water:
Metals react with water to form metal hydroxides and hydrogen.
Eg. Sodium reacts with water to form sodium hydroxide and hydrogen.
2Na + 2H2O → 2NaOH + H2
E.g. Magnesium reacts with water to form magnesium hydroxide and hydrogen.
Mg + H2O → Mg(OH)2+ H2
Reaction with acids:
Metals react with acids to form metallic salts and hydrogen.
Eg. Zinc reacts with dilute hydrochloric acid to form zinc chloride and hydrogen.
Zn + 2HCl → ZnCl2+ H2
Metals replace metals:
A more reactive metal replaces a less reactive metal from its salt solution.
Eg. Magnesium replaces copper from copper sulphate solution to form magnesium sulphate and copper.
Mg + CuSO4 → MgSO4+ Cu
Zinc replaces copper from copper sulphate solution to for zinc sulphate and copper.
Zn + CuSO4 → ZnSO4 + Cu
8: State some of the chemical properties of non-metals.
Answer:
Reaction with oxygen:
Non-metals react with oxygen to form non-metallic oxides. These oxides are acidic oxides because they react with water to form acids.
Eg. Sulphur burns in air to form sulphur dioxide. Sulphur dioxide reacts with water to form sulphurous acid.
S + O2 → SO2
SO2+ H2O → H2SO3
Reaction with water:
Non-metals do not react with water
Reaction with acids:
Most non-metals do not react with acids.
Some non-metals like sulphur react with concentrated nitric acid to form sulphur dioxide, nitrogen dioxide and water.
S + 4HNO3→ SO2 + 4NO2+ 2H2O
9: Explain the process of rusting of iron.
Answer: The surface of some metals gets corroded when exposed to moist air for a long time. This is called corrosion. Rusting of iron is an example of corrosion, here an oxide is formed and the chemical equation for rusting of iron is
2Fe + 3/2 O2 → Fe2O3.xH2O
10: Explain the process of rusting of copper.
Answer: When a copper vessel is exposed to moist air for long time, it acquires a dull green coloured coating on its surface; this green material is the mixture of copper hydroxide and copper carbonate. Reaction is as follows:
2Cu+H2O+CO2+O2→ Cu(OH)2 + CuCO3
11: Explain reaction between sulphur and oxygen. What is the nature of its oxide formed?
Answer: Non-metals react with oxygen to form non-metallic oxides. These oxides are acidic oxides because they react with water to form acids.
Eg. Sulphur burns in air to form sulphur dioxide. Sulphur dioxide reacts with water to form sulphurous acid.
S + O2 → SO2
SO2+ H2O → H2SO3
12: Explain activity series of metals.
Answer: The arrangement of metals in decreasing order of their reactivity is called reactivity series of metals. Reactivity series of metals is used to summarize information about the reactions of metals with acids and water, single displacement reactions and the extraction of metals from their ores.
13: Explain noble metals and give examples.
Answer: Noble metals are those metals which are not reactive while some are less reactive. There are certain metals that do not react with oxygen, water, moisture or even any of the dilute acids. We can imply that they are very unreactive. For example: gold and platinum.
14: State the role played by metals in our daily life.
Answer: Metals plays very important role in our day to day life, metals like iron is used for making pins, nuts, nails, bolts tools, machines etc.
1. Aluminium is used for making utensils, wires, parts of aircraft, vehicle, packaging of food stuffs and medicines etc.
2. Copper is used for making wires, vessels and electrical gadgets.
3. Gold and silver are used in making jewellery, coins and medals and platinum is used for making metals which are used for making jewellery, electrical gadgets, etc.
4. Sodium compounds are used as common salt, calcium compounds are used for making cement, glass etc.
15: State the role played by non-metals in our daily life.
Answer: Non-metals are essential for our life. Non-metals like oxygen are used for respiration by living beings and for burning of fuels. Nitrogen is used for making ammonia which is an important constituent of fertilizers used for improving soil quality for crop production. Sulphur is used for making sulphuric acid and salts of metals. Hydrogen is used for making ammonia which is used for making fertilizers and as fuels in rockets. Chlorine is used to kill germs in water and iodine is used as an antiseptic as well as a disinfectant.
16: State all possible ways of prevention of corrosion of metals.
Answer: Corrosion of metals can be prevented by:
- Applying oil grease
- Applying paint
- Galvanisation
- Electroplating
- Alloying: (Eg. When iron is alloyed with chromium and nickel, it forms stainless steel which is resistant to corrosion)
17: Give an example to illustrate that generally metallic oxides are basic in nature.
Answer: Burn a magnesium ribbon in air, and analyse the ash which is formed. The ash obtained by burning magnesium ribbon is dissolved in water and tested for its acidic or basis nature, we will observe it turns red litmus blue, thus concluded oxides of magnesium is basic in nature, amd in general metallic oxides are basic in nature.
18: Have you ever seen a greenish deposit on the surface of copper vessels, what is that?
Answer: When a copper vessel is exposed to moist air for long time, it acquires a dull green coloured coating on its surface;this green material is the mixture of copper hydroxide and copper carbonate. Reaction is as follow:
2Cu+H2O+CO2+O2 → Cu(OH)2 + CuCO3
19: Explain how metals and non-metals react with water.
Answer: Metals react with water to form metal hydroxides and hydrogen.
Eg. Sodium reacts with water to form sodium hydroxide and hydrogen.
2 Na + 2 H2O → 2 NaOH + H2
Magnesium reacts with water to form magnesium hydroxide and hydrogen.
Mg + H2O → Mg(OH)2+ H2
Non-metals do not react with water
20: What do you mean by displacement reaction?
Answer: In a displacement reaction a more reactive metal can replace a less reactive metal, but a less reactive metal cannot replace a more reactive metal. For e.g. Iron replaces copper from copper sulphate solution to form iron sulphate and copper
Fe + CuSO4 →FeSO4+ Cu
21: Write down the physical properties of metals.
Answer: Physical properties of metals:
- Metals are solid except mercury.
- Metals are hard.
- Metals are malleable that is can be beaten into thin sheets.
- Metals are ductile that is can be drawn into wires.
- Metals produce ringing sounds are called sonorous.
- Metals are lustrous.
- Metals are good conductors of heat and electricity.
22: Write down the physical properties of non-metals.
Answer: Physical properties of non-metals:
- Non- metals are solid, liquid or gas.
- Non- metals which is solid are brittle (diamond is the hardest).
- Non- metals are soft and dull in appearance.
- Non- metals are not malleable, and break down into powdery mass on tapping with a hammer.
- They are not sonorous.
- They are poor conductors of heat and electricity.
23. Have you ever seen a blacksmith beating an iron piece? Do you find a change in the shape of these pieces on beating? Would you expect a similar change in wood log on beating?
Answer: Yes, we have seen blacksmith beating the iron pieces. We have seen the changes in the shape on beating. It increases in size and it does not break.
If a wood log is beaten, it does not change its shape but it breaks into pieces.
24. What is malleability? Name two most malleable metals.
Answer: We see that the shape of iron and aluminium and other metals changes on beating. This property of metals due to which they can be beaten into thin sheets is called malleability. Silver and gold are the most malleable metals.
25. What happens when a magnesium ribbon is heated in presence of air?
Answer: When a magnesium ribbon is heated in presence of air on a burner flame, after some time it starts burning with a white flame and white powder is formed which is called magnesium oxide.
Mg (Magnesium) + O2 (Oxygen) → MgO (Magnesium Oxide)
26. Explain an activity to test the nature of rust.
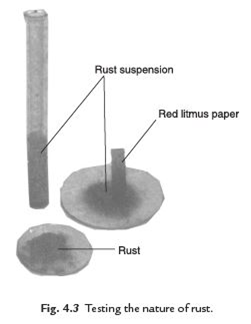
Answer: Collect some amount of rust after a reaction between iron, oxygen and water. Dissolve it in a very little amount of water. Shake well the mixture of rust and water. Test the solution with the red and blue litmus papers. We observe that the red litmus paper turns into blue. It shows that the nature of rust is basic.
27. Explain the reaction of sodium and water with the help of an activity.
Answer: Take a beaker, fill it half with water. Cut a small piece of sodium metal. Dry it using filter paper and wrap it in a small piece of cotton. Put the piece of sodium, wrapped in cotton into the beaker. We observe that the beaker becomes hot. We test the solution with red and blue litmus papers. It turns red litmus into blue. This activity indicates that sodium is highly reactive and it reacts vigorously with water. A lot of heat is generated in the reaction to form basic solution of sodium hydroxide.

Long Answer Type Questions
1: Explain physical and chemical properties of metals and non-metals.
Answer: Chemical properties of metals:
Reaction with oxygen:
Metals react with oxygen to form metallic oxides. These are basic oxides because they react with water to form bases.
Eg. Magnesium burns in air to form magnesium oxide.
2 Mg + O2 → 2 MgO
Reaction with water:
Metals react with water to form metal hydroxides and hydrogen.
Eg. Sodium reacts with water to form sodium hydroxide and hydrogen.
2Na + 2H2O → 2NaOH + H2
E.g. Magnesium reacts with water to form magnesium hydroxide and hydrogen.
Mg + H2O → Mg(OH)2+ H2
Reaction with acids:
Metals react with acids to form metallic salts and hydrogen.
Eg. Zinc reacts with dilute hydrochloric acid to form zinc chloride and hydrogen.
Zn + 2HCl → ZnCl2+ H2
Metals replace metals:
A more reactive metal replaces a less reactive metal from its salt solution.
Eg. Magnesium replaces copper from copper sulphate solution to form magnesium sulphate and copper.
Mg + CuSO4 → MgSO4+ Cu
Zinc replaces copper from copper sulphate solution to for zinc sulphate and copper.
Zn + CuSO4 → ZnSO4 + Cu
Chemical properties of non-metals:
Reaction with oxygen:
Non-metals react with oxygen to form non-metallic oxides. These oxides are acidic oxides because they react with water to form acids
Eg. Sulphur burns in air to form sulphur dioxide. Sulphur dioxide reacts with water to form sulphurous acid.
S + O2 → SO2
SO2+ H2O → H2SO3
Reaction with water:
Non-metals do not react with water
Reaction with acids:
Most non-metals do not react with acids.
Some non-metals like sulphur react with concentrated nitric acid to form sulphur dioxide, nitrogen dioxide and water.
S + 4HNO3→ SO2 + 4NO2+ 2H2O
Physical properties of metals:
- Metals are solid except mercury.
- Metals are hard.
- Metals are malleable that is can be beaten into thin sheets.
- Metals are ductile that is can be drawn into wires.
- Metals produce ringing sounds are called sonorous.
- Metals are lustrous.
- Metals are good conductors of heat and electricity.
Physical properties of non-metals:
- Non- metals are solid, liquid or gas.
- Non- metals which is solid are brittle (diamond is the hardest).
- Non- metals are soft and dull in appearance.
- Non- metals are not malleable, and break down into powdery mass on tapping with a hammer.
- They are not sonorous.
- They are poor conductors of heat and electricity..
2: Give reason why copper cannot displace zinc from its salt solution, and why sodium and potassium are stored in kerosene.
Answer: Copper cannot displace zinc from its salt solution: because Zinc is more reactive than copper. A more reactive metal can replace a less reactive metal, but a less reactive one cannot replace a more reactive metal. Therefor Copper cannot displace zinc from its salt solution.
Sodium and potassium are stored in kerosene because Sodium metal is very reactive. It reacts vigorously with oxygen and water. A lot of heat is generated in the reaction. It is, therefore, stored in kerosene.
3: Write word equation of the following reactions:
(a) Sulphur burning in air
(b) Sulphur reacts with concentrated acid
(c) Iron nail placed in copper sulphate solution.
Answer: (a) Sulphur burning in air: Sulphur burns in air to form sulphur dioxide. Sulphur dioxide reacts with water to form sulphurous acid.
S + O2→ SO2
SO2+ H2O→ H2SO3
(b) Sulphur reacts with concentrated acid to form sulphur dioxide, nitrogen dioxide and water.
S + 4HNO3 → SO2+ 4NO2+ 2H2O
(c) When Iron nail is placed in copper sulphate solution iron replaces copper from copper sulphate, thus nails becomes copper plated and blue colour of copper sulphate solution disappears and solutions turns from blue to light yellowish. Following reaction occurs:
Copper sulphate (CuSO4) + Iron nail (Fe) → Iron sulphate (FeSO4) + Copper (Cu)
4: What happens when dilute sulphuric acid is poured on a copper plate?
Answer: When dilute sulphuric acid is poured in a copper plate then copper undergo reaction with acid to form metal salts CuSO4 a blue crystalline solid and hydrogen gas:
Cu (Copper) + H2SO4 (Sulphuric Acid) → CuSO4(Copper sulphate) + H2 (Hydrogen gas)