Extra Questions for Class 10 Science Chapter 1 Chemical Reactions And Equations
Get extra questions for Class 10 Science Chapter 1 Chemical Reactions And Equations with PDF. Our subject expert prepared these solutions as per the latest NCERT textbook. These extra questions will be helpful to revise the important topics and concepts. You can easily download all the questions and answers in PDF format from our app.
Chemical Reactions And Equations Class 10 Science Extra Questions with Answers
Question 1: State one basic difference between a physical change and a chemical change.
Answer: In physical change, no new substance is formed, whereas in a chemical change, new substance(s) is/are formed.
Question 2: What is meant by a chemical reaction?
Answer: The reaction representing a chemical change is called a chemical reaction.
For example:
AgNO3(aq) + NaCl(aq) → AgCl(s)4↓ + NaNO3(aq)
FeS + H2SO4 → FeSO4 + H2S↑
↑ shows the gas is evolved whereas ↓ shows insoluble substance (precipitate) is formed.
Question 3: Hydrogen being a highly inflammable gas and oxygen being a supporter of combustion, yet water which is a compound made up of hydrogen and oxygen is used to extinguish fire. Why?
Answer: It is because properties of compound (H2O) are different from properties of its constituting elements, i.e. H2 and O2.
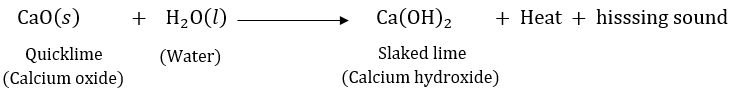
Question 4: What happens chemically when quicklime is added to water filled in a bucket?
Answer: Quicklime reacts with water to form slaked lime (calcium hydroxide) and produces a lot of heat and hissing sound.
Question 5: On what basis is a chemical equation balanced?
Answer: A chemical reaction is balanced on the basis of law of conservation of mass.
Question 6: What change in colour is observed when white silver chloride is left exposed to sunlight? State the type of chemical reaction in this change.
Answer: Silver chloride becomes grey. It is a photochemical decomposition reaction.
Question 7: Write a balanced chemical equation for the reaction between sodium chloride and silver nitrate indicating the physical state of the reactants and the products.
Answer: AgNO3 (aq) + Nacl(aq) → AgCl(s) + NaNO3 (aq)
Question 8: In electrolysis of water, why is the volume of gas collected over one electrode double that of gas collected over the other electrode?
Answer: It is because water contains hydrogen and oxygen in the ratio of 2:1.
Question 8: “We need to balance a skeletal chemical equation.” Give reason to justify the statement.
Answer: Skeletal chemical equations are unbalanced. We need to balance chemical equation because of law of conservation of mass. It states that ‘matter can neither be created nor be destroyed’. Therefore, chemical equation must be balanced in each and every chemical reaction.
Question 9: (i) Write a balanced chemical equation for the process of photosynthesis.
(ii)When do desert plants take up carbon dioxide and perform photosynthesis?
Answer:
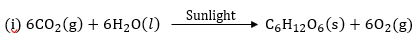
(ii) In desert plants the stomata are open at night. They take CO2 at night and is stored in the form of acid and is used during day time for photosynthesis.
Question 10: What is observed when a solution of potassium iodide solution is added to a solution of lead nitrate? Name the type of reaction. Write a balanced chemical equation to represent the above chemical reaction.
Answer: Yellow precipitate of lead iodide is formed. It is precipitation reaction.
Pb(NO3)2 (aq) + 2KI (aq) → Pbl2 (s) + 2KNO3 (aq)
It is also called double displacement reaction.
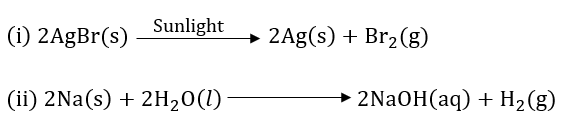
Question 11: Write balanced chemical equations for the following reactions.
(i) Silver bromide on exposure to sunlight decomposes into silver and bromine,
(ii) Sodium metal reacts with water to form sodium hydroxide and hydrogen gas.
Question 12: Identify the type of reaction(s) in the following equations.
(i) CH4 + 2O2 → CO2 + 2H2O
(ii) Pb(NO3)2 + 2KI → Pbl2 + 2KNOs
(iii) CaO + H2O → Ca(OH)2
(iv) CuSO4 + Zn → ZnSO4 + Cu
Answer: (i) Combustion reaction and oxidation reaction.
(ii) Double displacement reaction and precipitation reaction.
(iii) Combination reaction.
(iv) Displacement reaction.
Question 13: Write balanced equation for the reaction between magnesium and hydrochloric acid. Name the product obtained, identify the type of reaction.
Answer: Mg(s) + 2HCl(dil.) → MgCl2 (aq) + H2 (g)
The product formed is magnesium chloride and hydrogen gas. It is a displacement reaction.
Question 14: What is the colour of ferrous sulphate crystals? How does this colour change after heating?
Answer: The colour of ferrous sulphate is pale green. The colour changes to reddish brown on heating due to formation of iron (III) oxide.
Question 14: Why does the colour of copper sulphate solution change when an iron nail is dipped in it? Write two observations.
Answer: It is because displacement reaction takes place. Iron displaces copper from copper sulphate solution and forms pale green coloured solution of FeSO4 and reddish brown copper metal gets deposited.
Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
Question 15: Translate the following statement into chemical equation and then balance it Barium chloride reacts with aluminium sulphate to give aluminium chloride and a precipitate of barium sulphate. State the two types in which this reaction can be classified.
Answer: 3BaCl2(aq) + Al2(SO4)3(aq) → 3BaSO4(s) + 2AlCl3(aq)
It can be classified as double displacement as well as precipitation reaction.
Question 16: Why are decomposition reactions called the opposite of combination reactions? Write equations for these reactions.
Answer: In decomposition reaction, a compound is broken down into simpler compounds or elements, e.g.

Combination reaction is a reaction in which two or more elements or compounds combine to form a new compound, e.g.
N2 (g) + 3H2(g) → CuO(s) + CO2 (g)
Thus, decomposition and combination reactions are opposite to each other.
Question 17: (a) A solution of substance ‘X’ is used for white washing. What is the substance ‘X’? State the chemical reaction of ‘X’ with water.
(b) Why does the colour of copper sulphate solution change when an iron nail is dipped in it?
Answer: (a) ‘X’ is calcium oxide (CaO).
CaO(s) + H2O(l) → Ca(OH)2(aq) + heat
(b) It is because iron displaces copper from CuSO4 to form FeSO4 which is pale green.
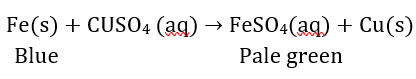
Question 18: A zinc plate was put into a solution of copper sulphate kept in a glass container. It was found that blue colour of the solution gets fader and fader with the passage of time. After few days, when zinc plate was taken out of the solution, a number of holes were observed on it.
(i) State the reason for changes observed on the zinc plate.
(ii) Write the chemical equation for the reaction involved.
Answer: (i) It is because zinc has displaced copper from CuSO4. Zinc metal has been used to form zinc sulphate, therefore, number of holes were observed.

Question 19: A white salt on heating decomposes to give brown fumes and a residue is left behind.
(i) Name the salt.
(ii) Write the equation for the decomposition reaction.
Answer: (i) Lead nitrate is white salt.

Question 20: When a solution of potassium iodide is added to a solution of lead nitrate in a test tube, a reaction takes place.
(a) What type of reaction is this?
(b) Write a balanced chemical equation to represent the above reaction.
Answer: (a) Double displacement as well as precipitation reaction.

Question 21: Define combination reaction. Give one example of a combination reaction which is also exothermic.
Answer: A reaction in which two elements or compounds combine to form a single compound is called combination reaction.
CaO(s) + H2O(l) → Ca(OH)2(aq) + Heat
It is also an exothermic reaction along with a combination reaction because heat is evolved.
Question 22: (a) Classify the following reactions into different types.
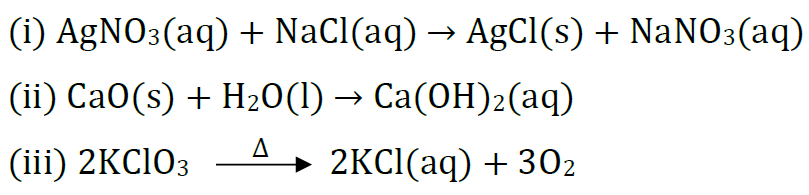
(b) Which of the above reaction(s) is/are precipitation reaction(s)? Why is a reaction called precipitation reaction?
Answer: (a) (i) Precipitation reaction (Double displacement reaction)
(ii) Combination reaction
(iii) Decomposition reaction
(b) Reaction (i) is a precipitation reaction because one of the product formed is insoluble in water.
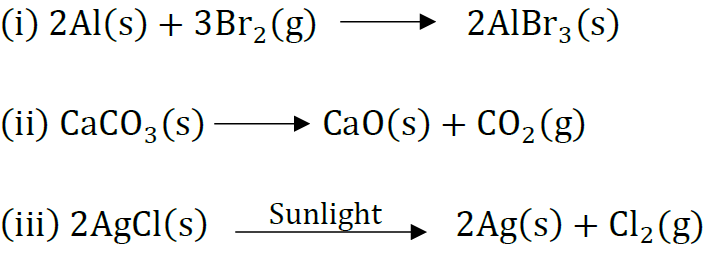
Question 23: Write balanced equations for the following mentioning the type of reaction involved.
(i) Aluminium + Bromine → Aluminium bromide
(ii) Calcium carbonate → Calcium oxide + Carbon dioxide
(iii) Silver chloride → Silver + Chlorine
Answer:
Question 24: (a) Why is respiration considered as an exothermic reaction?
(b) Define the terms oxidation and reduction.
(c) Identify the substance that is oxidised and reduced in the following reaction.
CuO(s) + Zn(s) → Cu(s) + ZnO(s)
Answer: (a) It is because heat is evolved during respiration.
(b) Oxidation is a process in which O2 is added or H2 is removed or loss of electrons take place. Reduction is a process in which H2 is added or O2. is removed or gain of electrons take place.
(c) Zn is getting oxidised, CuO is getting reduced.
Question 25: What is meant by
(i) precipitation reaction,
(ii) exothermic reaction,
(iii) oxidation reaction?
Write balanced chemical equations for an example of each.
Answer: (i) Precipitation reaction: The reaction in which two compounds exchange their ions and the product formed is insoluble in water is called precipitation reaction.
AgNO3 (aq) + KI (aq) → AgI (s) + KNO3 (aq)
(ii) Exothermic reaction: The reaction in Which heat is evolved is known as exothermic reaction.
C+O2 → CO2 + heat
(iii) Oxidation reaction: The reaction in which O2 is added or H2 is removed or loss of electrons takes place is called oxidation reaction.
2Cu + O2 → 2CuO
Question 26: You might have noted that when copper powder is heated in a china dish, the surface of copper powder becomes coated with a black colour substance.
(i) How has this black coloured substance formed?
(ii) What is that black substance?
(iii) Write the chemical equation of the reaction that takes place.
Answer: (i) Copper reacts with oxygen to form copper oxide which is black, i.e. oxidation of copper takes place.
(ii) Copper oxide
(iii) 2Cu(s) + O2(g) → 2CuO(s)
Question 27: What happens when an aqueous solution of sodium sulphate reacts with an aqueous solution of barium chloride? State the physical conditions of reactants in which the reaction between them will not take place. Write the balanced chemical equation for the reaction and name the type of reaction.
Answer: White precipitate of barium sulphate is formed. If both reactants are in solid state, then the reaction will not take place between them.
BaCl2(aq) + Na2SO4(aq) → BaSO4(s) + 2NaCl(aq)
It is a double displacement as well as a precipitation reaction.
Question 28: What is a redox reaction? When a magnesium ribbon burns in air with a dazzling flame and forms a white ash, is magnesium oxidised or reduced? Why?
Answer: The reactions in which oxidation (loss of electrons) and reduction (gain of electrons) take place simultaneously are called redox reactions.
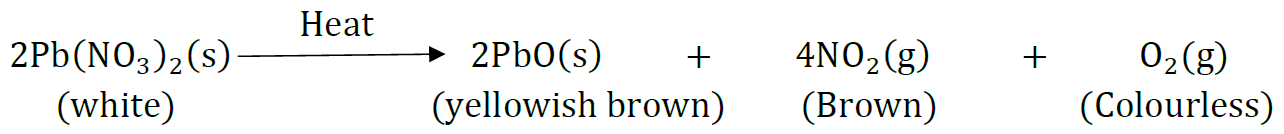
Question 29: Write any two observations in an activity which may suggest that a chemical reaction has taken place. Give an example in support of your answer.
Answer: Any two of these observations will suggest chemical reaction has taken place.
(i) Change in state.
(ii)Change in colour.
(iii) Evolution of gas.
(iv) Change in temperature.
For example, lead nitrate is white crystalline solid which on heating gives yellowish brown solid (lead monoxide). A brown gas and a colourless gas is also evolved. It shows chemical reaction has taken place.
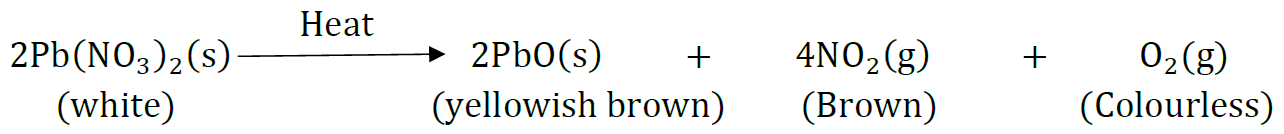
Question 30: When the powder of a common metal is heated in an open china dish, its colour turns black. However, when hydrogen is passed over the hot black substance so formed, it regains its original colour. Based on the above information, answer the following questions.
(i) What type of chemical reaction takes place in each of the two given steps?
(ii) Name the metal initially taken in the powder form. Write balanced chemical equations for both reactions.
Answer: (i) In first step, oxidation takes place. In second step, redox reaction takes place.
(ii) Metal in the powder form is copper.
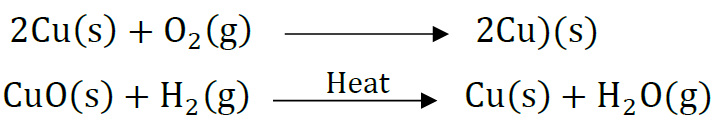
Question 31: Name the products formed on strongly heating ferrous sulphate crystals. What type of chemical reaction occurs in this change?
Answer:
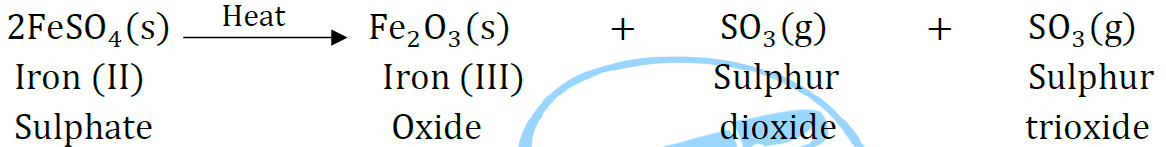
It is decomposition reaction.
Question 32: What is an oxidation reaction? Give an example of oxidation reaction. Is oxidation an exothermic or an endothermic reaction?
Answer: The reaction in which oxygen or electronegative element is added or hydrogen or electropositive element is removed or loss of electrons takes place, is called an oxidation reaction. For example:
Fe2+ → Fe3+ + e‒ (loss of electron)
C + O2 → CO2 + Heat
Oxidation reactions are mostly exothermic in nature because heat is evolved in this process.
Question 33: Distinguish between a displacement reaction and a double displacement reaction.
Answer: Displacement reaction is a reaction in which more reactive metal can displace less reactive metal from its salt solution.
Double displacement reaction are those reactions in which compounds exchange their ions to form two new compounds.
Question 34: When you have mixed the solutions of lead(II) nitrate and potassium iodide,
(i) what was the colour of the precipitate formed and can you name the precipitate?
(ii) write the balanced chemical equation for this reaction.
(iii) is this also a double displacement reaction?
Answer: (i) The colour of the precipitate is yellow. The name of compound formed as a precipitate is Pbl2 (lead iodide).

Question 35: What do you mean by exothermic and endothermic reactions? Give examples.
Answer: Exothermic reactions are those in which heat is evolved, e.g.
C(s) + O2(g) → CO2 + heat
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) + heat
Endothermic reactions are those reactions in which heat is absorbed, e.g.
Question 36: A Name the type of chemical reaction represented by the following equation:
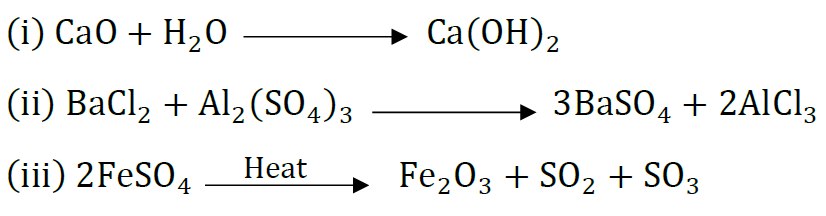
Answer: (i) Combination reaction
(ii) Double displacement reaction (Precipitation reaction)
(iii) Decomposition reaction.
Question 37: Write the chemical equation of the reaction in which the following changes have taken place with an example of each:
(i) Change in colour
(ii) Change in temperature
(iii) Formation of precipitate
Answer: (i) Cu(s) + 2AgNO3 (aq) → Cu(NO3)2(aq) + 2Ag
The solution will become blue in colour and shiny silver metal will be deposited.
(ii) NaOH + HCl → NaCl + H2O+ heat
The temperature will increase because heat will be evolved.
(iii) Pb(NO3)2 (aq) + 2KI (aq) → Pbl2 (s) + 2KNO3 (aq)
Yellow precipitate of Pbl2 will be formed.
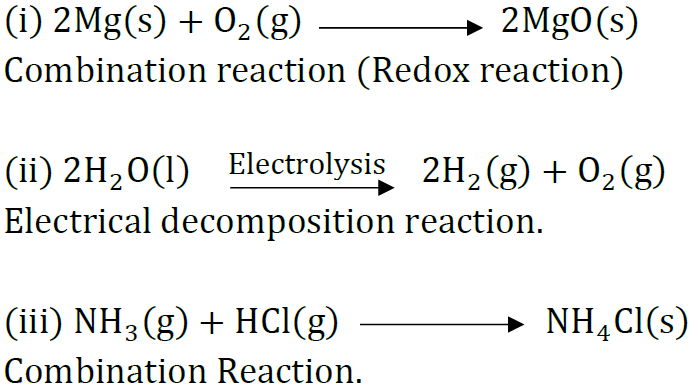
Question 38: State the type of chemical reactions and chemical equations that take place in the following:
(i) Magnesium wire is burnt in air.
(ii) Electric current is passed through water.
(iii) Ammonia and hydrogen chloride gases are mixed.
Answer:
Question 39: (a) Write the essential condition for the following reaction to take place:
2AgBr → 2Ag + Br2
Write one application of this reaction.
(b) Complete the following chemical equation of a chemical reaction 2FeSO4
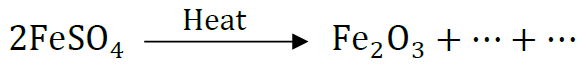
(c) What happens when water is added to quick line. Write chemical equation.
Answer:
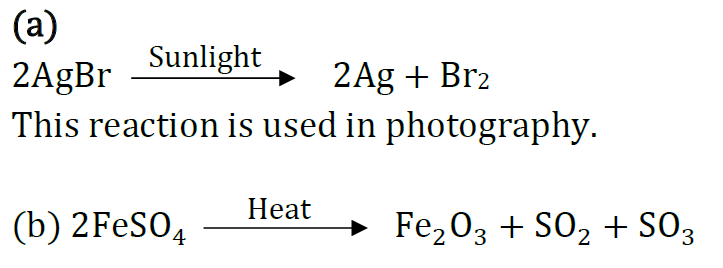
(c) Slaked lime is formed with hissing sound and lot of heat is evolved.
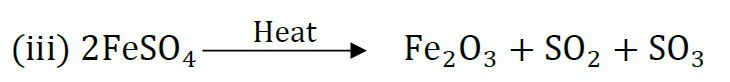
Question 40: 2g of ferrous sulphate crystals are heated in a dry boiling tube.
(i) List any two observations.
(ii) Name the type of chemical reaction taking place.
(iii) ‘Write the chemical equation for the reaction.
Answer: (i) (a) Green colour of FeSO4 disappears and reddish brown solid is formed.|
(b) Smell of burning sulphur.
(ii) Decomposition reaction
Question 41: Write chemical equation reactions taking place when carried out with the help of
(a) Iron reacts with steam
(b) Magnesium reacts with dil HCl
(c) Copper is heated in air.
Answer:
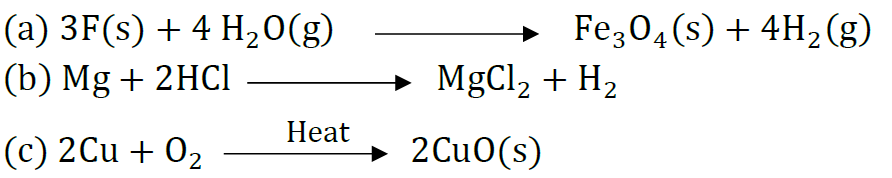
Question 42: Which products will be obtained when lead nitrate is heated simply. Write balanced chemical equation for the reaction? State the type of chemical reaction that occur in the change.
Answer: Lead monoxide, nitrogen dioxide and oxygen gas will be liberated.
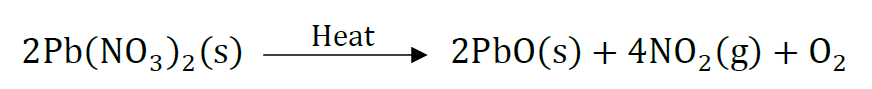
It is thermal decomposition reaction.
Question 43: What is meant by skeletal type chemical equation? What does it represent? Using the equation for electrolytic decomposition of water, differentiate between a skeletal chemical equation and a balanced chemical equation.
Answer: The equations in which gaseous are written in atomic form instead of molecular form and equation is not balanced, are called skeletal type equation. They represent gaseous elements formed in atomic state and the equation is not balanced.
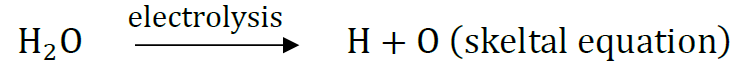
Hydrogen and oxygen are written in atomic forms and equation is not balanced.
H2O → H2 + O2 is also skeletal equation
2H2O → 2H2 + O2 is balanced equation
Question 44: What is rancidity? Mention any two ways by which rancidity can be prevented.
Answer: The process in which taste and smell of food gets spoiled is called rancidity. It happens due to oxidation. Prevention from rancidity:
(i) Antioxidants are added to fatty acids to prevent oxidation, e.g. chips are packed in presence of nitrogen gas which prevents spoilage by oxidation.
(ii) Food should be kept in airtight container in refrigerator.
Question 45: Write balanced chemical equation for the reactions that take place during respiration. Identify the type of combination reaction that takes place during this process and justify the name. Give one more example of this type of reaction.
Answer: C6H12O6 + 6O2 → 6CO2 + 6H2O + heat
It is an exothermic combination reaction because heat is evolved.
CH4(g) + 2O2(g) → CO2 (g) + 2H2O
Combustion of methane is another example of exothermic combination reaction.
Question 46: What is redox reaction? Identify the substance oxidised and the substance reduced in the following reactions.
(i) 2PbO + C → 2Pb + CO2
(ii) MnO2 + 4HCl → MnCl2 + 2H2O + Cl2
Answer: Those reactions in which oxidation and reduction takes place simultaneously are called redox reactions.
(i) PbO is getting reduced and C is getting oxidised.
(ii) MnO2 is getting reduced and HCl is getting oxidised.
Question 47: Write the balanced chemical equations for the following reactions and identify the type of reaction in each case.
Thermite reaction, iron (III) oxide reacts with aluminium and gives molten iron and aluminium oxide.
Answer:
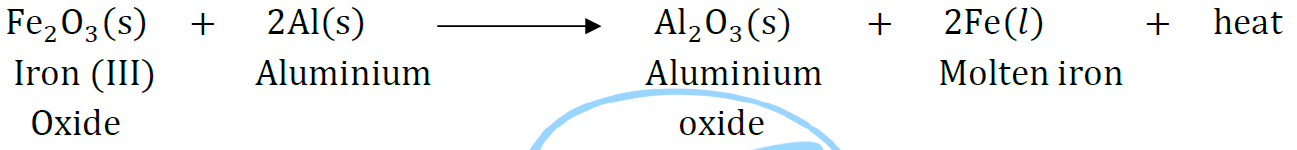
It is a displacement reaction because Al is displacing Fe from Fe2O3.
Molten iron is used for repairing broken railway tracks.
Question 48: A solution of potassium chloride when mixed with silver nitrate solution, an insoluble white substance is formed. Write the chemical reaction involved and also mention the type of the chemical reaction?
Answer:
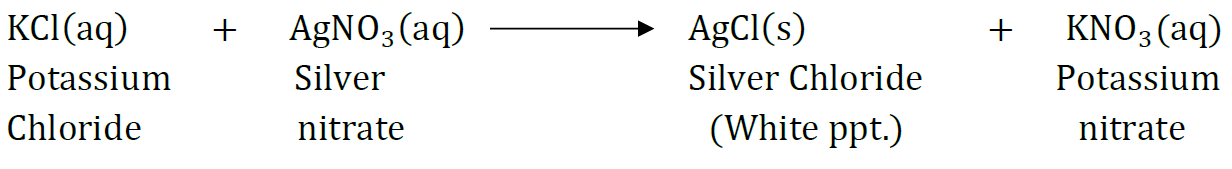
It is a double displacement reaction. It is also a precipitation reaction as AgCl is a white precipitate.
Question 49: (a) Define a balanced chemical equation. Why should an equation be balanced?
(b) Write the balanced chemical equation for the following reaction:
(i) Phosphorus burns in presence of chlorine to form phosphorus penta chloride.
(ii) Burning of natural gas.
(iii) The process of respiration.
Answer: (a) Balanced chemical equation has an equal number of atoms of different elements in the reactants and products. According to law of conservation of mass, matter can neither be created nor be destroyed in a chemical reaction.
(b) (i) P4 (s) + 10Cl2 (g) → 4PCl5 (S)
(ii) CH4 (g) + 2O2 (g) → CO2 (g) + 2H2O(l) + heat energy
(iii) C6H12O6 (s) + 6O2 (g) + 6H2O → 6CO2 (aq) + 12H2O (l) + energy
Question 50: (a) Explain two ways by which food industries prevent rancidity.
(b) Discuss the importance of decomposition reaction in metal industry with three points.
Answer: (a) (i) Rancidity can be prevented by adding antioxidants to food containing fat and oil, e.g. butylated hydroxy anisole is added to butter as antioxidant.
(ii) It can be prevented by packaging fat and oil containing foods in nitrogen gas.
(b) (i) Molten NaCl is electrolytically decomposed to form sodium metal.
(ii) Aluminium metal is obtained by electric decomposition of bauxite ore mixed with cryolite.
(iii) Carbonate ores are thermally decomposed to give metal oxide which on reduction give metal.
Question 51: (a) Write one example for each of decomposition reaction carried out with help of
(i) Electricity (ii) Heat (iii) Light
(b) Which of the following statements is correct and why copper can displace silver from silver nitrate and silver can displace copper from copper sulphate solution.
Answer:
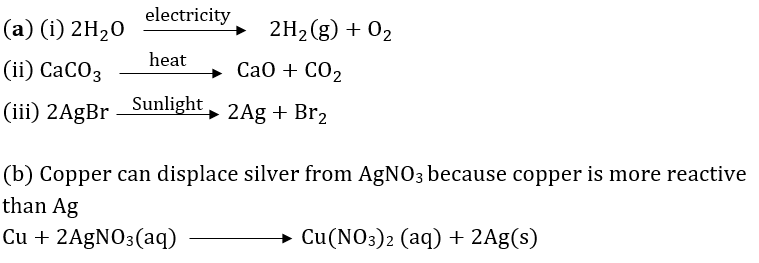
(b) Copper can displace silver from AgNO3 because copper is more reactive than Ag
Cu + 2AgNO3(aq) → Cu(NO3)2 (aq) + 2Ag(s)