Extra Questions for Class 10 Science Chapter 2 Acids, Bases And Salts
Get extra questions for Class 10 Science Chapter 2 Acids, Bases And Salts with PDF. Our subject expert prepared these solutions as per the latest NCERT textbook. These extra questions will be helpful to revise the important topics and concepts. You can easily download all the questions and answers in PDF format from our app.
Acids, Bases And Salts Class 10 Science Extra Questions with Answers
Question 1: The pH of a sample of vegetable soup was found to be 6.5. How is this soup likely to taste?
Answer: The taste will be slightly sour as it is weakly acidic.
Question 2: Which bases are called alkalies? Give an example of alkalies.
Answer: Soluble bases are called alkalies, e.g. sodium hydroxide (NaOH).
Question 3: Write a balanced chemical equation for the reaction between sodium carbonate and hydrochloric acid indicating the physical state of the reactants and the products.
Answer: Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + CO2(g) + H2O(l)
Question 4: Write a balanced chemical equation for a neutralisation reaction, mentioning the physical state of the reactants and the products.
Answer: NaOH(s) + 2HCl(aq) → NaCl(aq) + H2O(l)
Question 5: What would be the colour of red litmus in a solution of sodium carbonate?
Answer: The red litmus will change to blue in sodium carbonate solution.
Question 6: Which gas is evolved when sodium hydrogencarbonate reacts with dilute hydrochloric acid?
Answer: Carbon dioxide gas is evolved.
Question 7: Curd is not kept in copper and brass utensils. Why?
Answer: Curd and sour substances contain acids which react with brass and copper vessels to form poisonous salts which are harmful for our health.
Question 8: Name the gas usually liberated when a dilute acid reacts with a metal. What happens when a burning candle is brought near this gas?
Answer: H2 gas is liberated. It burns with pop sound when burning candle is brought near the gas.
Question 9: What effect does an increase in concentration of H+(aq.) in a solution have on the pH of solution?
Answer: Higher the concentration, lower will be pH of the solution.
Question 10: Why does 1 M HC1 solution have a higher concentration of H+ ions than 1 M CH3COOH solution?
Answer: 1 M HCl has higher cone, of (H+) because it ionises completely in aqueous solution whereas CH3COOH does not as it is weak acid.
Question 11: Which gas is generally liberated when a dilute solution of hydrochloric acid reacts with an active metal?
Answer: Hydrogen gas is liberated when active metal reacts with dilute hydrochloric acid.
Zn(s) + 2HCl (dil.) → ZnCl2(aq) + H2(g)
Question 12: What is the colour of litmus in a solution of ammonium hydroxide?
Answer: Red litmus will turn blue in ammonium hydroxide.
Question 13: How will you test for the gas which is liberated when hydrochloric acid reacts with an active metal?
Answer: Bring a burning matchstick near the gas. It burns with ‘pop’ sound showing that it is hydrogen.
Question 14: Name the acid present in the following:
(i) Tomato (ii) Vinegar (iii) Tamarind
Answer: (i) Oxalic acid (ii) Acetic acid (iii) Tartaric acid
Question 15: mL of water and 10 mL of sulphuric acid are to be mixed in a beaker
(i) State the method that should be followed with reason.
(ii) What is this process called?
Answer: (i) The acid is to be added slowly in water to prevent the mixture to be splashed. The reaction is highly exothermic therefore, constant cooling should be done.
(ii) The process is called dilution.
Question 16: Explain how antacid works.
Answer: Hyperacidity is caused by excess of hydrochloric acid in stomach. Antacid is basic in nature. It neutralizes excess of acid and gives relief from pain caused by hyperacidity.
Question 17: Name the natural source of each of the following acid
(i) Citric acid.
(ii) Oxalic acid.
(iii) Lactic acid.
(iv) Tartaric acid.
Answer: (i) Lemon and orange.
(ii) Tomatoes and Guava.
(iii) Sour milk (curd)
(iv) Tamarind.
Question 18: A student detected the pH of four unknown solution A, B, C and D as follows 11, 5, 7 and 2. Predict the nature of the solution.
Answer: A is basic ‘B’ is acidic ‘C’ is natural and ‘D’ is strongly acidic.
Question 19: State the chemical name of Plaster of Paris. Write a chemical equation to show the reaction between Plaster of Paris and water.
Answer: Calcium sulphate hemihydrate.
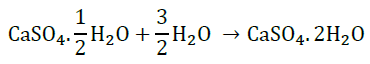
Question 20: State in brief the preparation of washing soda from baking soda. Write balanced chemical equation of the reaction involved.
Answer: Sodium hydrogencarbonate (baking soda) on heating gives sodium carbonate which on recrystallisation gives washing soda.
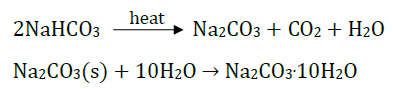
Question 21: What is the colour of FeSO4.7H2O crystals? How does this colour change upon heating? Give balanced chemical equation for the changes.
Answer: Pale green is the colour of FeSO4.7H2O crystals. It becomes dirty white on heating.
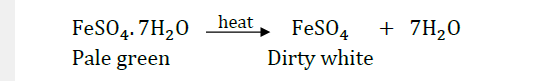
Question 22: Classify the following salts into acidic, basic and neutral: Potassium sulphate, ammonium chloride, sodium carbonate, sodium chloride.
Answer: Neutral: Potassium sulphate, Sodium chloride
Acidic: Ammonium chloride
Basic: Sodium carbonate
Question 23: A student dropped few pieces of marble in dilute HC1 contained in a test tube. The evolved gas was passed through lime water.
(i) What change would be observed in lime water?
(ii) Write balanced chemical equation for the above change.
Answer: (i) Lime water will turn milky due to formation of calcium carbonate.
(ii) Ca(OH)2 (aq) + CO2 (g) → CaCO3 (s) + H2O(l)
Question 24: What happens when chlorine is passed over slaked lime at 313K? Write chemical equation of the reaction involved and state two uses of the product obtained.
Answer: Bleaching powder is formed.

(i) It is used as bleaching agent in paper and textile industries.
(ii) It is used as disinfectant in purification of drinking water
Question 25: What is meant by ‘water of crystallisation’ of a substance?
Answer: The water molecules associated with a crystalline substance is called ‘water of crystallisation’.
Question 26: (a) Define olfactory indicators. Name two substances which can be used as olfactory indicator.
(b) Choose strong acids from the following:
CH3COOH, H2SO4, H2CO3, HNO3
Answer: (a) Those substances whose smell (odour) changes in acidic or basic solution are called olfactory indicators, e.g. onion and vanilla.
(b) H2SO4 and HNO3 are strong acids.
Question 27: Explain the action of dilute hydrochloric acid on the following with chemical equation:
(i) Magnesium ribbon (ii) Sodium hydroxide (iii) Crushed egg shells
Answer: (i) Hydrogen gas will be formed
Mg(s) + 2HCl (dil) → MgCl2 (aq) + H2 (g)
(ii) Sodium chloride and water will be formed
NaOH + HCl → NaCl + H2O
(iii) Crushed egg shell are made up of CaCO3 which reacts with dil HCl to give brisk effervescence due to CO2
CaCO3(s) + 2HCl CaCl2 + H2O + CO2
Question 28: (i) Give the constituents of baking powder
(ii) Why cake or bread swells on adding baking powder? Write chemical equation.
Answer: (i) Baking powder containing sodium hydrogen carbonate and tartaric acid.
(ii) It is due to carbon dioxide
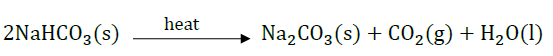
Question 29: (a) Write the name given to bases that are highly soluble in water. Give an example.
(b) How is tooth decay related to pH? How can it be prevented?
(c) Why does bee sting cause pain and irritation? Rubbing of baking soda on the sting area gives relief. How?
Answer: (a) Alkali, e.g. NaOH (Sodium hydroxide).
(b) Lower the pH, more will be tooth decay. Acid reacts with Ca3(PO4)2 and cause tooth decay. It can be prevented by brushing teeth after every meal.
(c) It is due to formic acid. Sodium hydrogencarbonate (Baking soda) neutralises formic acid giving relief.
Question 30: A white powder is added while baking breads and cakes to make them soft and fluffy. Write the name of the powder. Name its main ingredients. Explain the function of each ingredient. Write the chemical reaction taking place when the powder is heated during baking.
Answer: Baking powder.
It consists of sodium hyrogencarbonate and tartaric acid.
Sodium hydrogencarbonate gives CO2 which makes cake soft and fluffy. Tartaric acid neutralizes the bitterness due to sodium carbonate produced.
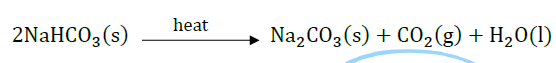
Question 31: “Sodium hydrogencarbonate is a basic salt”. Justify the statement. How is it converted into washing soda? Explain.
Answer: Sodium hydrogencarbonate is a salt of sodium hydroxide (strong base) and carbonic acid (weak acid).
It is basic salt. It is converted into washing soda by heating followed by crystallization.
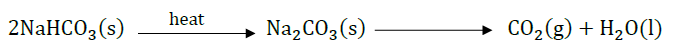
Question 32: (a) What is universal indicator?
(b) Write the chemical equation involved in the preparation of sodium hydroxide. Name the process.
Answer: (a) Universal indicator is the mixture of synthetic indicators which is used to find pH of solutions.
Question 33: A gas ‘X’ reacts with lime water and forms a compound ‘Y’ which is used as a bleaching agent in chemical industry. Identify ‘X’ and ‘Y\ Give the chemical equation of the reactions involved.
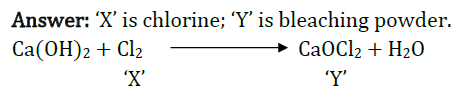
Question 34: (i) Name the compound which is obtained from baking soda and is used to remove permanent hardness of water.
(ii) Write its chemical formula.
(iii) What happens when it is recrystallised from its aqueous solution?
Answer: (i) Sodium carbonate is obtained from baking soda and is used to remove hardness of water.
(ii) Na2CO3
(iii) It changes to washing soda, Na2CO3.10H2O
Question 35: What is a neutralisation reaction? Give two examples.
Answer: The reaction between acid and base to form salt and water is called neutrilisation reaction. Examples:
KOH + HNO3 → KNO3 + H2O
2NaOH + H2SO4 → Na2SO4 + 2H2O
Question 36: What is tooth enamel chemically? State the condition when it starts corroding. What happens when food particles left in the mouth after eating degrade? Why do doctors suggest use of tooth powder/toothpaste to prevent tooth decay?
Answer: It is made up of calcium phosphate. It starts corroding due to acid formed in mouth. The food particles which are left in mouth form acids which cause tooth decay. Toothpaste and tooth powder are basic and neutralise acid formed in mouth which prevents tooth decay.
Question 37: What is Plaster of Paris chemically? How is it prepared? List its two important uses.
Answer: Calcium sulphate hemihydrate.
It is prepared by heating gypsum at 373 K.
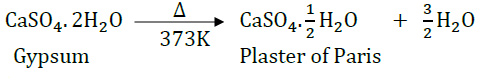
(i) It is used to prepare chalks.
(ii) It is used to make casts and moulds.
Question 38: What is baking soda chemically called? Give reaction involved in its preparation. Write one of its uses.
Answer: Sodium hydrogencarbonate.
NH3 + CO2 + H2O + NaCl → NaHCO3 + NH4Cl
OR
NaCO3 + CO2 + H2O → 2NaHCO3
It is used as an antacid.
Question 39: (a) What is an alkali? Give an example.
(b) Why do HCl, HNO3, etc. show acidic characters in aqueous solutions while solutions of compounds like alcohol and glucose do not show acidic character?
Answer: (a) Soluble bases are called alkalies, e.g. sodium hydroxide is an alkali.
(b) HCl, HNO3 ionise in aqueous solution, whereas alcohol and glucose do not show acidic characters because they do not ionise in aqueous solution.
Question 40: A compound which is prepared from gypsum has the property of hardening when mixed with proper quantity of water.
(i) Identify the compound.
(ii) Write the chemical equation for its preparation.
(iii) Mention one important use of this compound.
Answer: (i) Plaster of Paris
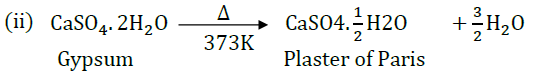
(iii) It is used for plastering fractured bones.
Question 41: Name the products formed in each case when
(a) hydrochloric acid reacts with caustic soda.
(b) granulated zinc reacts with caustic soda.
(c) carbon dioxide is passed into lime water.

Question 42: State reason for the following statements:
(i) Tap water conducts electricity whereas distilled water does not.
(ii) Dry hydrogen chloride gas does not turn blue litmus red whereas dilute hydrochloric acid does.
(iii) During summer season, a milk man usually adds a very small amount of baking soda to fresh milk.
(iv) For a dilution of acid, acid is added into water and not water into acid.
(v) Ammonia is a base but does not contain hydroxyl group.
Answer: (i) Tap water contains ions which conduct electricity, distilled water does not contain ions.
(ii) Dry HCl does not form ions but HCl gives H+ and Cl–.
(iii) Baking soda does not allow milk to change to lactic acid which makes milk sour.
(iv) Adding water to acid is highly exothermic. Therefore, water is added to acid very slowly with cooling.
(v) Ammonia dissolves in water and forms H– Therefore, it is basic in nature.
Question 43: (a) State the chemical properties on which the following uses of baking soda are based:
(i) as an antacid
(ii) as a soda acid fire extinguisher
(iii) to make bread and cake soft and spongy.
(b) How is washing soda is obtained from baking soda? Write balanced chemical equation.
Answer: (a) (i) It is weakly basic in nature and naturalize hyperacidity.
(ii) It liberates CO2 with H2SO4, which extinguish fire.
(iii) It liberates CO2 on heating which makes bread and cake soft and sponge
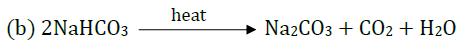
Baking soda on heating gives sodium carbonate which on crystallisation from hydrated washing soda.
Na2CO3 + 10H2O → Na2CO3.10H2O