Extra Questions for Class 10 Science Chapter 5 Periodic Classification Of Elements
Get extra questions for Class 10 Science Chapter 5 Periodic Classification Of Elements with PDF. Our subject expert prepared these solutions as per the latest NCERT textbook. These extra questions will be helpful to revise the important topics and concepts. You can easily download all the questions and answers in PDF format from our app.
Periodic Classification Of Elements Class 10 Science Extra Questions with Answers
Question 1: Lithium, sodium and potassium form a Dobereiner’s triad. The atomic masses of lithium and potassium are 7 and 39 respectively. Predict the atomic mass of sodium.
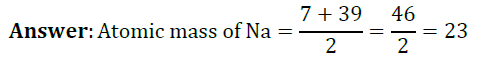
Question 2: Chlorine, bromine and iodine form a Dobereiner’s triad. The atomic masses of chlorine and iodine are 35.5 and 126.9 respectively. Predict the atomic mass of bromine.
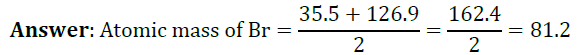
Question 3: Why was the system of classification of elements into triads not found suitable?
Answer: It is because all the elements discovered at that time could not be classified into triads.
Question 4: The elements of the second period of the Periodic Table are given below:
Li Be B C N O F
(a) Give reason to explain why atomic radii decrease from Li to F.
(b) Identify the most
(i) metallic and
(ii) non-metallic element.
Answer: (a) It is because nuclear charge increases due to increase in atomic number, therefore, force of attraction between nucleus and valence electrons increases, i.e. effective nuclear charge increases, hence atomic radii decrease from Li to F.
(b) (i) Most metallic element is ‘Li’ as it can lose electrons easily due to larger atomic size.
(ii) Most non-metallic element is ‘F’ because it can gain electrons easily due to the smallest atomic size.
Question 5: The elements of the third period of the Periodic Table are given below:
| Group | I | II | III | IV | V | VI | VII |
| Period 3 | Na | Mg | Al | Si | P | S | Cl |
(a) Which atom is bigger, Na or Mg? Why?
(b) Identify the most (i) metallic and (ii) non-metallic element in Period 3.
Answer: (a) Sodium is bigger than magnesium as it has lesser nuclear charge so there is less force of attraction between nucleus and valence electrons and less effective nuclear charge. It is, therefore, bigger in size.
(b) (i) Sodium is the most metallic as it can lose electrons easily due to its larger atomic size,
(ii) Chlorine is the most non-metallic element because it can gain electrons easily due to its smallest atomic size.
Question 6: State Mendeleev’s periodic law. Write two achievements of Mendeleev’s periodic table
Answer: Mendeleev’s Periodic Law: ‘Properties of elements are the periodic function of their atomic masses.
Achievements:
- It could classify all the elements discovered at that time.
- It helped in discovery of new elements.
- It helped in correction of atomic mass of some of the elements.
Question 7: How can the valency of an element be determined if its electronic configuration is known? What will be the valency of an element of atomic number 9 (nine)?
Answer: If the element has 1, 2, 3, 4 valence electrons, its valency will be 1, 2, 3, 4 respectively. If the element has 5, 6, 7, 8 valence electrons, its valency will be 3, 2, 1, 0. Element with atomic number 9 has electronic configuration 2, 7. So, its valency will be 1.
Question 8: How does the electronic configuration of an atom of an element relate to its position in the modern periodic table? Explain with one example.
Answer: The position of element depends upon number of valence electrons which depend upon electronic configuration. Those elements which have same valence electrons, occupy same group. Those elements which have one valence electron belong to group 1.
Elements with two valence electrons belong to group 2.
Period number is equal to number of shells.
If valence electrons are equal to 1, it belongs to group 1. If it has 2 shells, it belongs to second period, e.g. if element ‘X’ has atomic number 11, its electronic configuration is 2, 8,1. It has one valence electron, it belongs to group 1 and it has three shells therefore, it is in third period.
Question 9: The atomic numbers of three elements, X, Y and Z are 9,11 and 17 respectively. Which two of these elements will show similar chemical properties? Why?
Answer: Electronic configuration of X, Y and Z will be:
X(9) : 2, 7
Y(11) : 2, 8, 1
Z(17) : 2, 8, 7
X and Z will show similar chemical properties due to same number of valence electrons.
Question 10: On the basis of electronic configuration, how will you identify the first and the last element of a period?
Answer: First element has 1 valence electron and last element has 8 valence electrons. Number of shells remain the same in the same period.
Question 11: In the modern periodic table, the element Calcium (atomic number = 20) is surrounded by elements with atomic numbers 12, 19, 21 and 38. Which of these elements has physical and chemical properties resembling those of Calcium and why?
Answer: Elements with atomic number 12, 38 resemble calcium in physical and chemical properties because they have same number of valence electrons and belong to same group 2.
Mg(12) : 2, 8, 2
Ca(20) : 2, 8, 8, 2
Sr(38) : 2, 8, 18, 8, 2
Question 12: How does the metallic character of elements change along a period of the periodic table from the left to the right and why?
Answer: The metallic character goes on decreasing along a period from left to right because atomic size goes on decreasing therefore, tendency to lose electrons decreases.
Question 13: How does the valency of elements vary
(a) in going down a group, and
(b) in going from left to right in a period of the periodic table?
Answer: (a) Valency remains the same in a group.
(b) Valency first goes on increasing from left to right in a period till middle of period, then decreases.
Question 14: In the periodic table, how does the tendency of atoms to lose electrons change on going from
(i) left to right across a period?
(ii) top to bottom in a group?
Answer: (i) Tendency to lose electrons decreases from left to right across a period.
(ii) Tendency to lose electrons increases from top to bottom in a group.
Question 15: Give reasons:
(i) Elements in a group have similar chemical properties.
(ii) Elements of Group I form ions with a charge of +1.
Answer: (i) Elements in a group have same number of valence electrons and same valency therefore have similar chemical properties.
(ii) It is because elements of group 1 lose one electron to acquire +1 charge and become stable.
Question 16: An element ‘X’ has atomic number 13.
(a) Write its electronic configuration.
(b) State the group to which ‘X’ belongs.
(c) Is ‘X’ a metal or a non-metal?
(d) Write the formula of its bromide.
Answer:
(a) 2, 8, 3
(b) Group 13
(c) ‘X’ is a metal.
(d) XBr3, XBr3 is formula of its bromide.
Question 17: State the Modern Periodic Law for classification of elements. How many
(a) groups and (b) periods are there in the Modern Periodic Table?
Answer: ‘Properties of elements are the periodic function of their atomic number.’
(a) There are 18 groups and
(b) 7 periods in the Modern Periodic Table.
Question 18: An element ‘M’ has atomic number 11.
(a) Write its electronic configuration.
(b) State the group to which ‘M’ belongs.
(c) Is ‘M’ a metal or a non-metal?
(d) Write the formula of its chloride.
Answer:
(a) 2, 8, 1
(b) Group 1
(c) ‘M’ is a metal.
(d) ‘MCL’ is formula of its chloride.
Question 19: the formula of its oxide an element ‘M’ has atomic number 12.
(a) Write its electronic configuration.
(b) State the group to which ‘M’ belongs.
(c) Is ‘M’ a metal or a non-metal?
(d) Write the formula of its oxide.
Answer:
(a) 2, 8, 2
(b) Group 2
(c) ‘M’ is a metal.
(d) MO is the formula of its oxide.
Question 20: An element ‘X’ belongs to 3rd period and group 17 of the periodic table. State its
(a) electronic configuration, (b) valency. Justify your answer with reasoning.
Answer: (a) X(17): 2, 8, 7
(b) Valency: 1
It has atomic number 17 and therefore, electronic configuration will be 2, 8, 7. It can gain one electron to become stable therefore, its valency is equal to 1. It belongs to third period, as it has three shells. It belongs to group 17 because it has 7 valence electrons.
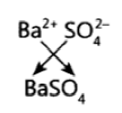
Question 21: The formula of magnesium oxide is MgO. State the formula of barium nitrate and barium sulphate, if barium belongs to the same group as magnesium.
Answer: Barium Nitrate – Ba(NO3)2
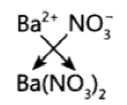
Barium Sulphate – BaSO4
Question 22: Choose from the following:
20Ca, 3Li, 11Na, 10Ne
(a) An element having two shells completely filled with electrons.
(b) Two elements belonging to the same group of the periodic table.
Answer: (a) 10Ne has electronic configuration 2, 8. Its both shells are completely filled.
(b) 3Li and 11Na belong to same group of periodic table i.e., 1st group.
Question 23: Why do all the elements of the (a) same group have similar properties, (b) same period have different properties?
Answer: (a) Elements of same group have similar properties due to same number of valence electrons, therefore, they have same valency.
(b) Elements of same period have different properties as they differ in number of valence electrons.
Question 24: An element ‘E’ has following electronic configuration:
| K | L | M |
| 2 | 8 | 6 |
(a) To which group of the periodic table does element ‘E’ belong?
(b) To which period of the periodic table does element ‘E’ belong?
(c) State the number of valence electrons present in element ‘E’.
(d) State the valency of the element ‘E’.
Answer:
(a) ‘E’ belongs to group 16.
(b) It belongs to 3rd period.
(c) It has 6 valence electrons.
(d) Its valency is equal to 2.
Question 25: Choose from the following:
4Be, 9F, 19K, 20Ca
(a) The element having one electron in the outermost shell.
(b) Two elements of the same group.
Answer: (a) 19K has one valence electron.
(b) 4Be and 20Ca belong to the same group.
Question 26: An element has atomic number 13.
(a) What is the group and period number to which this element belongs?
(b) Is this element a metal or a non- metal? Justify your answer.
Answer: (a) It belongs to group 13 and 3rd period.
(b) It is a metal because it can lose 3 electrons to become stable.
Question 27: The electronic configuration of two elements ‘A’ and ‘B’ are 2, 8, 3 and 2, 8, 7 respectively. Find the atomic number of these elements. State the nature and formula of the compound formed by the union of these two elements.
Answer:
Answer: ‘A’ has atomic number 13.
‘B’ has atomic number 17.
It is an ionic compound.
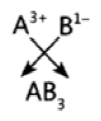
Question 28: The atomic number of three elements are given below:
| Element (symbol) | A | B | C |
| Atomic Number | 5 | 7 | 10 |
Write the symbol of the element which belongs to (o) group 13, (b) group 15, of the periodic table. State the period of the periodic table to which these elements belong. Give reason for your answer.
Answer: (a) A belongs to group 13 because its electronic configuration is 2, 3, ie. it has 3 valence electrons.
(b) ‘B’ belongs to group 15 because its electronic configuration is 2, 5, ie. it has 5 valence electrons. They belong to 2nd period as they both have two shells.
Question 29: The atomic numbers of three elements are given below:
| Element (symbol) | A | B | C |
| Atomic Number | 3 | 6 | 9 |
Write the symbol of the element which belongs to (a) group 1, (b) group 14, of the periodic table. State the period of the periodic table to which these elements belong. State reason to support your answer.
Answer: (a) A belongs to group 1.
(b) B belongs to group 14.
These elements belong to second period because these elements have two shells. A has electronic configuration 2, 1 and has one valence electron so, belongs to group 1. ‘B’ has electronic configuration 2, 4 and has four valence electrons so, belongs to group 14 and period 2. ‘C’ has electronic configuration 2, 7 and has one valence electron. All of them contain two shells and so belong to second period.
Question 30: The position of three elements A, B and C in the Periodic Table is shown below:
| Group 16 | Group 17 |
| ― | ― |
| ― | A |
| ― | ― |
| B | C |
Giving reasons, explain the following:
(a) Element A is a non-metal.
(b) Element B has a larger atomic size than element C.
(c) Element C has a valency of 1
Answer: (a) ‘A’ is non-metal because it can gain electron easily as it has 7 valence electrons and forms negative ion with stable electronic configuration.
(b) It is because ‘B’ has lesser atomic number, less nuclear charge, less force of attraction between valence electrons and nucleus therefore, has larger atomic size.
(c) ‘C’ has 7 valence electrons. It can gain one electron to become stable. So, its valency is equal to one.
Question 31: The position of three elements A, B and C in the Periodic Table is shown below:
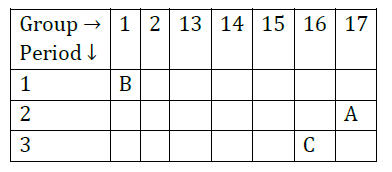
Giving reasons, explain the following:
(a) Element A is non-metal.
(b) Atom of element C has a larger size than atom of element A.
(c) Element B has a valency of 1.
Answer: (a) It is because it has 7 valence electrons.
It can gain one electron to form negative ion. So, it is a non-metal.
(b) ‘C’ has more number of shells than A. So, it is larger in size.
(c) ‘B’ has one valence electron. It can lose one electron to become stable. So, its valency is equal to 1.
Question 32: What physical and chemical properties of elements were used by Mendeleev in creating his periodic table? List two observations which posed a challenge to Mendeleev’s Periodic Law.
Answer: Atomic mass as a physical property and nature and formulae of oxide and hydride formed, and chemical property was used by Mendeleev.
Following are the two observations which posed a challenge to Mendeleev’s Periodic Law.
(i) Increasing order of atomic weights could not be maintained while matching chemical properties. Chemical properties do not depend upon atomic mass.
(ii) Isotopes have different atomic mass but same chemical properties.
Question 33: Table given below shows a part of the Periodic Table.
(b) Atomic size of Mg is less than that of Na.
(c) Fluorine is more reactive than Chlorine.
Answer: (a) They can lose electrons easily due to bigger size; energy required to remove electron is less.
(b) It is because of greater effective nuclear charge on Mg, i.e. more number of protons attract more number of electrons than Na.
(c) ‘F’ can form F― more easily than Cl due to smaller atomic size. F―s is more stable than Cl―. Therefore, fluorine is more reactive than chlorine.
Question 34: (a) Why do we classify elements?
(b) What were the two criteria used by Mendeleev in creating his Periodic Table?
(c) Why did Mendeleev leave some gaps in his Periodic Table?
(d) In Mendeleev’s Periodic Table, why was there no mention of Noble gases like Helium, Neon and Argon?
(e) Would you place the two isotopes of chlorine, CI―35 and CI―37 in different slots because of their different atomic masses or in the same slot because their chemical properties are the same? Justify your answer.
Answer: (a) It is done so as to study the properties of elements conveniently.
(b) Increasing order of atomic mass and similarities in chemical properties (especially nature and formulae of oxide and hydride formed).
(c) These gaps were left for undiscovered elements.
(d) Noble gases were not invented at that time.
(e) They will be kept at same slot as they have same chemical properties.
Question 35: (a) What is meant by periodicity in properties of elements with reference to the periodic table?
(b) Why do all the elements of the same group have similar properties?
(c) How will the tendency to gain electrons change as we go from left to right across a period? Why?
Answer: (a) The repetition of same properties after definite interval is called periodicity in properties.
(b) It is because they have same valence electrons therefore, have similar properties.
(c) Tendency to gain electrons increases from left to right in a period because atomic size goes on decreasing and effective nuclear charge increases.
Question 36: (a) What are ‘groups’ and ‘periods’ in the ‘periodic table’?
(b) Two elements M and N belong to group I and II respectively and are in the same period of the periodic table. How do the following properties of M and N vary?
(i) Sizes of their atoms
(ii) Their metallic characters
(iii) Their valencies in forming oxides
(iv) Molecular formulae of their chlorides
Answer: (a) The vertical columns in the periodic table are called ‘groups’. The horizontal rows in the periodic table are called ‘periods’.
(b) (i) ‘M’ and ‘N’ belong to same period but group I and II. Therefore, ‘N’ will be smaller than ‘M’ as atomic size goes on decreasing from left to right.
(ii) ‘M’ is more metallic than ‘N’. Metallic character goes on decreasing from left to right as tendency to lose electrons decreases due to decrease in atomic size.
(iii) Their valencies are 1 and 2 respectively in forming oxides. Valency goes on increasing first and then decreases.
(iv) MCI, NCI2 are molecular formulae of their chlorides.
Question 37: Atoms of seven elements A, B, C, D, E, F and G have a different number of electronic shells but have the same number of electrons in their outermost shells. The elements A and C combine with chlorine to form an acid and common salt respectively. The oxide of element A is liquid at room temperature and is a neutral substance, while the oxides of the remaining six elements are basic in nature. Based on the above information answer the following questions: What could the element A be?
(i) Will elements A to G belong to the same period or same group of the periodic table?
(iii) Write the formula of the compound formed by the reaction of the element A with oxygen,
(iv) Show the formation of the compound by a combination of element C with chlorine with the help of electronic structure.
(v) What would be the ratio of number of combining atoms in a compound formed by the combination of element A with carbon?
(vi) Which one of the given elements is likely to have the smallest atomic radius?
Answer: (i)’A’ is hydrogen because its oxide H2O is liquid at room temperature.
(ii) A to G belong to same group of the periodic table as these have same number of valence electrons.
(iii) A2O
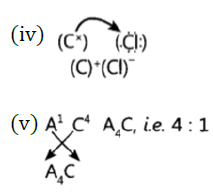
(vi) ‘A’ has smallest atomic size.
Question 38: In the following table, are given eight elements A, B, C, D, E, F, G and H (here letters are not the usual symbols of the elements) of the Modern Periodic Table with the atomic numbers of the elements in parenthesis.
| Period | Group 1 | Group 2 |
| 2 | A (3) | E (4) |
| 3 | B (11) | F (12) |
| 4 | C (19) | G (20) |
| 5 | D (37) | H (38) |
(i) What is the electronic configuration of F?
(ii) What is the number of valence electrons in the atom of F?
(iii) What is the number of shells in the atom of F?
(iv) Write the size of the atoms of E, F, G and H in decreasing order,
(v) State whether F is a metal or a non-metal.
(vi) Out of the three elements B, E and F, which one has the biggest atomic size?
Answer: (i) F has electronic configuration 2, 8, 2.
(ii) F has 2 valence electrons.
(iii) There are three shells in ‘F’.
(iv) H > G > F > E is decreasing order of size of atoms.
(v) ‘F’ is a metal.
(vi) ‘B’ is having the biggest atomic size among B, E and F.
Question 39: F, Cl and Br are the elements each having seven valence electrons. Which of these (a) has the largest atomic radius, (b) is most reactive? Justify your answer stating reason for each.
Answer: (a) Bromine has largest atomic radius because it has four shells: 2, 8, 18, 7.
(b) Fluorine is most reactive because it is smallest in size and can gain electron easily.
Question 40: Na, Mg and Al are the elements having one, two and three valence electrons respectively. Which of these elements (a) has the largest atomic radius, (b) is least reactive? Justify your answer stating reason for each.
Answer: (a) Na has the largest atomic radius because it has 11 protons and 11 electrons, therefore least effective nuclear charge.
(b) Al is least reactive because it has smallest atomic size due to 13 protons and 13 electrons, it has greater effective nuclear charge, therefore, cannot lose electrons easily, hence it is least reactive.
Question 41: (a) How many periods are there in the Modern Periodic Table of elements?
(b) How do atomic radius, valency and metallic character vary down a group?
(c) How do the atomic size and metallic character of elements vary as we move from left to right in a period?
Answer: (a) There are 7 periods.
(b) Atomic radius goes on increasing down the group, valency remains same. Metallic character increases down the group.
(c) Atomic size decreases along a period from left to right. Metallic character decreases along a period from left to right.