Carbon and its Compounds Class 10 Important Questions and Answers
Important Questions for Class 10 Science Chapter 4 Carbon and its Compounds covers each topic of the chapter. These questions aim at providing a better understanding of the chapter to the students and can be downloaded in PDF format. These important question bank help students in clearing their doubts so that they can score well in the exam.
While preparing for exams, students should practise these important questions of Class 10 Science to understand the concepts better. Solving important questions of Class 10 Science Chapter 4 will teach students time management skills and enhance their problem-solving skills. Also, students may come across a few of these questions in the board exam.
Important Questions for Class 10 Science Chapter 4 – PDF
1. Give the formulae of the following functional groups:
(a) Aldehyde
(b) Ketone
Answer: (a) – CHO
(b) – C = O
2. Give the electronic dot structure of CO2 molecule.
Answer: The electronic dot structure of CO2 is shown below.
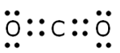
3. What is the unique ability of carbon atom?
Answer: Carbon has the ability of bond with other atoms of carbons forming large chains, branches or rings. This property is called catenation.
Also, carbon has a valency of four and can combine with other elements such as hydrogen, oxygen, nitrogen, etc. Due to its small size, the bonds formed by carbon with other elements are very strong. Hence, carbon forms a wide variety of compounds.
4. Silicon has same valence electrons like carbon but it do not show the property similar to carbon. Why?
Answer: The size of silicon atom is larger than that of carbon atom causing a reduced force of attraction between electrons and the nucleus. In other words, the compounds formed by silicon are very reactive whereas the compounds formed by the carbon are very stable. That is why silicon does not exhibit the property similar to carbon.
5. Where in the nature you can find the products of esterification reaction?
Answer: Ester is the product obtained from esterification reaction. These compounds are responsible for the pleasant smell of fruits. It is found in fruits such as apples, mangoes, pineapples, strawberries etc. Sometimes, esters are used as flavouring agents in food items and in perfumes as well.
6. What is the industrial application of hydrogenation?
Answer: In industries, hydrogenation is used to reduce vegetable oils to vanaspati ghee. Vanaspati ghee is used as a substitute for ghee or butter containing animal fats which are harmful to our health and is cheaper than butter. Vegetable oils are unsaturated and they are hydrogenated in the presence of catalysts like nickel or palladium.
7. Give the name of:
(a) HCOOCH3
(b) HCHO
Answer: (a) IUPAC name: Methyl methanoate
(b) IUPAC name: Methanal
8. What happens when ethanol gets oxidised? Name the type of oxidising agents used.
Answer: Ethanol undergoes oxidation to yield ethanoic acid. This oxidation is done with the help of strong oxidizing agents like alkaline potassium permanganate (KMnO4) and acidified potassium dichromate (K2Cr2O7) in the presence of heat.
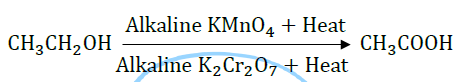
9. What happens when ethanol is heated at 443 K with excess of conc. H2SO4?
Answer: Concentrated sulphuric acid can act as a dehydrating agent. Hence, ethanol when heated at 443 K with excess conc. H2SO4 undergoes dehydration reaction to form ethene.
The reaction involved is:
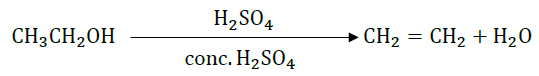
10. What is meant by esterification reaction? Give equation to explain the process.
Answer: Esterification is the process of producing an ester from the reaction of an alcohol and a carboxylic acid. An acid is used as the catalyst. The general reaction can be expressed as:
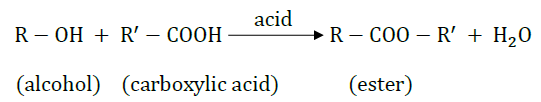
The –OH part of alcohol and the –H present in acid get removed and form water as the by product. The nomenclature of ester is of the form “alkyl alkanoate” where the alkyl part is derived from the alcohol and the alkanoate part is derived from the carboxylic acid.
11. What is meant by saponification? Explain the process and its practical utility.
Answer: Saponification is considered the reverse reaction of esterification. Esterification is the process of producing esters by the reaction of an alcohol and a carboxylic acid. Saponification is the process in which an ester upon hydrolysis in the presence of a base gives back the alcohol and the carboxylic acid. This process is called saponification because this is used in the preparation of soap. Soaps are sodium or potassium salts of long chain carboxylic acids.
12. Differentiate between a soap and a detergent.
Answer:
| Soaps | Detergents |
| Consist of a ‘-COONa’ group attached to a fatty acid having a long alkyl chain. | Consist of a ‘-SO3Na’ group attached to a long alkyl chain. |
| They are not effective in hard water and saline water | They do not lose their effectiveness in hard water and saline water. |
| Soaps are completely biodegradable | Detergents containing a branched hydrocarbon chain are non-biodegradable |
| They have a tendency to form sum in a hard water environment. | These compounds do not form scum. |
| They are derived from natural sources such as vegetable oils and animal fats. | Detergents are synthetic derivatives. |
| Soaps are environment-friendly products since they are biodegradable. | These compounds can form a thick foam that causes the death of aquatic life. |
| Examples of soaps: sodium palmitate and sodium stearate. | Examples of detergents: deoxycholic acid and sodium lauryl sulfate. |
13. What are addition reactions? Which category of compounds undergoes addition reactions? Explain.
Answer: Addition reactions are reactions in which two or more molecules combine to form a larger molecule, with no other products. No atoms or molecules get eliminated in this reaction. An example of addition reaction is the hydrogenation of alkenes to form alkanes.

Unsaturated compounds i.e., compounds containing double or triple bond(s) undergo addition reactions very easily. To accommodate the new incoming atom(s) (in the case of hydrogenation, the incoming atom is hydrogen), the double/triple bond(s) gets cleaved and converted into single bonds such that all elements present in the compound get a stable electronic configuration.
14. What is a homologous series? Explain with an example.
Answer: A series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called homologous series.
Consider the homologous series of alcohols – methanol, ethanol, etc. Note that methanol (CH3OH) is obtained by replacing one of the H atoms with –OH group. Similarly, the –OH group replaces one of the H atoms of ethane (CH3CH3) to form ethanol (CH3CH2OH) and so on. The chemical properties of these compounds are similar. The successive members of the homologous series differ by a –CH2 unit.
For example, CH3OH and CH3CH2OH, CH3CH2OH and CH3CH2CH2OH differ by a –CH2 unit.
Also, members of a homologous series can be represented using a general formula. In the case of alcohols, the general formula is CnH2n+1O, where ‘n’ is the number of carbon atoms.
15. Why scum is formed when soap is treated with hard water?
Answer: Scum is an insoluble precipitate formed due to the reaction of soap with calcium and magnesium salts present in hard water. As a result, soap does not form lather in hard water, making its cleansing action less effective in hard water.
16. Give only the mechanism of cleansing action of soaps.
Answer: Soaps are sodium or potassium salts of long chain carboxylic acids. They contain two ends having different properties: the carboxylic acid part which is hydrophobic and the ionic end (Na+ or K+) which is hydrophilic.
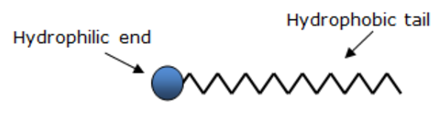
Structure of a soap molecule
Dirt present in clothes is basically a hydrocarbon. When soap is used in water, the soap molecules tend to surround the dirt such that they form a cluster in which the hydrophobic tails are in the interior of the cluster and the hydrophilic part are on the surface of the cluster (see figure below). The hydrophilic part dissolves in water and the hydrophobic part dissolves in the hydrocarbon (dirt). This formation or cluster is called micelle.
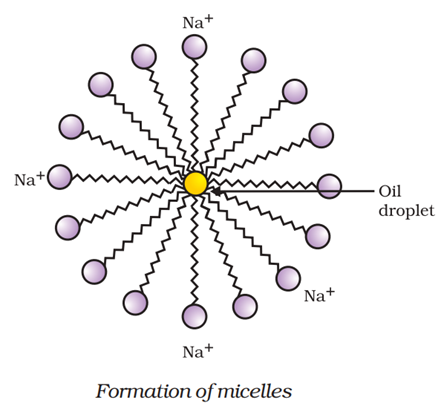
Agitating the water by hand or brush forces the hydrophilic end to move along the direction of agitation of water, dragging the micelle holding the dirt out of the clothes and the dirt is rinsed away. In short, the cleansing action of soap is achieved by the formation of micelles.
17. A compound of carbon which has acidic characteristic is used as preservative of pickles. This compound reacts with carbonates and bicarbonates to release a colourless gas. Identify the compound and the gas. Give equations for the reactions also.
Answer: The compound is ethanoic acid also known as acetic acid (CH3COOH). It is a weak organic acid. CH3COOH reacts with carbonates and bicarbonates to release CO2 which is a colourless gas. The reactions involved are:
2CH3COOH + Na2CO3→ 2CH3COONa + CO2↑ + H2O
CH3COOH + NaHCO3→ CH3COONa + CO2↑ + H2O
18. How would you distinguish experimentally between an alcohol and a carboxylic acid?
Answer: Alcohols can be distinguished from carboxylic acid using the sodium carbonate (Na2CO3) or sodium bicarbonate (NaHCO3) test. Alcohols do not react with sodium carbonate or bicarbonate, whereas acid reacts with them to release carbon dioxide.
2RCOOH + Na2CO3→ 2RCOONa + CO2↑ + H2O
RCOOH + NaHCO3→ RCOONa + CO2↑ + H2O
Alcohol + Na2CO3→ No reaction
Q.19. How an ethanol and ethanoic acid be differentiated on the basis of their physical and chemical properties?
Answer: Physical Properties:
Both Ethanol and ethanoic acid are liquids at room temperature and have distinct melting and boiling points.
| Physical Property | Ethanol | Ethanoic acid |
| Melting Point | 156K | 290K |
| Boiling Point | 351K | 391K |
Chemical Properties:
(i) Ethanoic acid produces CO2 when reacted with carbonates or bicarbonates. Ethanol does not react with carbonates or bicarbonates.
(ii) Ethanoic acid reacts with a base (say NaOH) to give salt and water:
CH3COOH + NaOH → CH3COONa + H2O
(iii) Ethanol reacts very slightly with NaOH.
20. How you can differentiate chemically between butter and cooking oil? What are substitution reactions? Explain giving a suitable example.
Answer: Butter is basically made of animal fats which contain saturated fatty acids. Cooking oil on the other hand, contains long unsaturated fatty acids. This means that vegetable oils can be hydrogenated whereas butter cannot be hydrogenated.
A reaction in which one atom or a group of atoms is replaced by another atom or another group of atoms is called substitution reaction. For example, consider the photochemical reaction of methane with chlorine. In the presence of sunlight, one of the H atoms is replaced by Cl atom. Gradually, each H gets replaced by Cl. The reaction can be represented by the equation:
CH4 + Cl2→ CH3Cl + HCl (in the presence of sunlight)
CH3Cl + Cl2→ CH2Cl2 + HCl and so on.
21. Give reason why:
(i) A mixture of ethyne and air is not used for welding whereas oxygen and ethyne mixture is preferred.
(ii) Carbon does not form C4+ and C4 – ions.
Answer: (i) When a mixture of air and ethyne (C2H2) is used, incomplete combustion takes place and as a result a sooty flame is obtained, which is not effective for welding.
If ethyne is burned with oxygen, complete combustion takes place and gives a clean flame which is ideal for welding. This welding is also known as oxy-acetylene welding.
(ii) Carbon has 4 valence electrons. The two given situations can be analysed separately.
To form C4+ cation, it has to lose 4 electrons, but an enormous amount of energy is required to remove the electrons, because of the strong nuclear force of attraction.
To form C4 – anion, it has to gain 4 electrons, but it would be difficult for the nucleus with 6 protons to hold onto 10 electrons. In brief, the anion formed would not be stable.