NCERT Solutions for Class 8 Science Chapter 6 Combustion and Flame
NCERT Solutions for Class 8 Science Chapter 6 Combustion and Flame are given below. Here we have provided the best and error-free answers to all the exercise questions that will strengthen your foundation in science. Solving NCERT questions will assist you in grasping the content in the Crop Production and Management chapter in a better way.
In these solutions, we have answered all the intext and exercise questions provided in NCERT class 8 science textbook. NCERT Solutions for Class 8 Science Chapter 6 Combustion and Flame provided in this article are strictly based on the CBSE syllabus and curriculum. Students can easily download these solutions in PDF format for free or can read them online.
Combustion and Flame Class 8 Science NCERT Solutions
Exercise Questions
Question 1: List conditions under which combustion can take place.
Answer: Conditions under which combustion can take place are as follows:
- Fuel is required
- Air (oxygen) is necessary.
- Ignition temperature is required.
Question 2: Fill in the blanks.
(a) Burning of wood and coal causes __________of air.
(b) A liquid fuel, used in homes is__________ .
(c) Fuel must be heated to its ____________ before it starts burning.
(d) The fire produced by oil cannot be controlled by___________ .
Answer: (a) Burning of wood and coal causes Pollution of air.
(b) A liquid fuel, used in homes is Kerosene.
(c) Fuel must be heated to its Ignition Temperature before it starts burning.
(d) The fire produced by oil cannot be controlled by Water.
Question 3: Explain how the use of CNG in automobiles has reduced pollution in our cities.
Answer: Combustion of fuels like petroleum causes formation of un-burnt carbon particles along with carbon monoxide gas. These harmful pollutants enter the air and cause respiratory diseases. Compressed Natural Gas (CNG) produces these harmful products in very less quantity. It is a comparatively cleaner fuel. Therefore, the use of CNG has reduced pollution in our cities.
Question 4: Compare LPG and wood as fuels
Answer:
| LPG | Wood |
| (i) It does not cause pollution on combustion. | (i) It pollutes air on its combustion. |
| (ii) No smoke is produced. | (ii) It produces smoke. |
| (iii) It is a liquid fuel. | (iii) It is a solid fuel. |
| (iv) It has more calorific value (55000 kJ/kg). | (iv) It has less calorific value (17000 kJ/kg). |
| (v) It can be easily transported, as it is stored in cylinders. | (v) It can’t be transported easily like LPG fuels. |
Question 5: Give reasons:
(a) Water is not used to control fires involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Answer: (a) Water is a good conductor of electricity. If it is used for controlling a fire involving electrical equipment, then the person dousing the fire might get an electric shock. Also, water can damage electrical equipment.
(b) LPG is a good fuel as compared to wood as it readily available and it is cheap. It burns easily in the air at a moderate rate and it produces a large amount of heat. It does not leave behind any undesirable substances.
(c) A piece of paper wrapped around aluminium pipe does not catch fire easily. This is because aluminium, being a metal, is a good conductor of heat. Therefore, heat is transferred from the paper to the metal and the paper does not attain its ignition temperature.
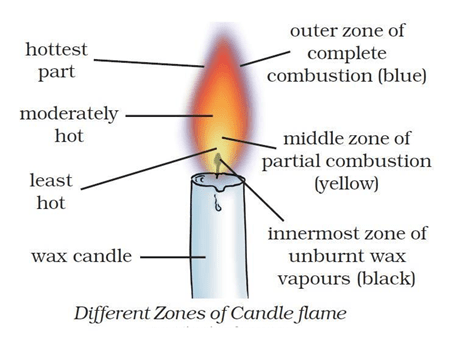
Question 6: Make a labelled diagram of a candle flame.
Answer:
Question 7: Name the unit in which the calorific value of a fuel is expressed.
Answer: The calorific value of a fuel is expressed in a unit called kilojoule per kg(kJ/kg).
Question 8: Explain how CO2 is able to control fires.
Answer: CO2 is a non-combustible gas and extinguishes fire in two ways:
(i) Since it is heavier than oxygen, it covers the fire like a blanket and cuts off the contact between oxygen and fuel.
(ii) In cylinders, CO2 is kept in the liquid form. When released, it expands enormously and cools down. This brings down the temperature of the fuel, which helps in controlling the fire.
Question 9: It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Answer: The green leaves hold some amount of water, so its ignition temperature gets increased and it does not burn easily. On the other hand, dry leaves are waterless, so they catch fire easily (having low ignition temperature)
Question 10: Which zone of a flame does a goldsmith use for melting gold and silver and why?
Answer: Goldsmith uses the outermost zone of the flame for melting gold and silver because it is the hottest zone (complete combustion) of the flame.
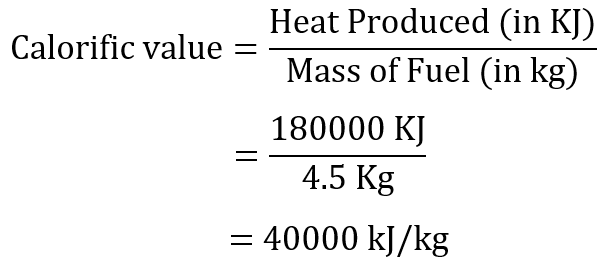
Question 11: In an experiment, 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Answer:
Question 12: Can the process of rusting be called combustion? Discuss.
Answer: No, as rusting is a very slow process as compared to combustion and the heat evolve in combustion is much more than rusting. Rusting can take place at room temperature but combustion needs an ignition temperature.
Question 13: Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time?
Answer: The water in Ramesh’s beaker will heat up in a shorter time. This is because the outermost zone of a flame is the hottest zone, while the yellow zone (in which Abida had kept the beaker) is less hot.
CBSE Class 8 NCERT solutions Science Chapter 6 PDF
Below we have listed the topics discussed in NCERT Solutions for Class 8 Science Chapter 6. The list gives you a quick look at the different topics and subtopics of this chapter.
| Section in NCERT Book | Topics Discussed |
|---|---|
| 6.1 | What is Combustion? |
| 6.2 | How Do We Control Fire? |
| 6.3 | Types of Combustion |
| 6.4 | Flame |
| 6.5 | Structure of a Flame |
| 6.6 | What is a Fuel? |
| 6.7 | Fuel Efficiency |
NCERT Solutions for Class 8 Science Chapter 6 – A Brief Discussion
CBSE Class 8 Science NCERT Solutions Chapter 6 helps students to clear their doubts and to score good marks in the board exam. All the questions are solved by experts with a detailed explanation that will help students complete their assignments & homework. Having a good grasp over CBSE NCERT Solutions for Class 8 Science will further help the students in their preparation for board exams and other competitive exams such as NTSE, Olympiad, etc.